The Fascinating World of Graphite: Properties, Applications, and Beyond
Podcast
Introduction
Graphite—often recognized as the “lead” in our pencils—has a much larger story to tell. This remarkable material, a crystalline form of carbon, is prized for its unique properties that make it indispensable in various industrial applications. In this post, we’ll explore the fundamentals of graphite, its distinct characteristics, and the diverse ways it impacts industries worldwide. From thermal and electrical conductivity to its applications in aerospace, batteries, and steelmaking, graphite proves to be a powerful material in the modern world.
1. What is Graphite?
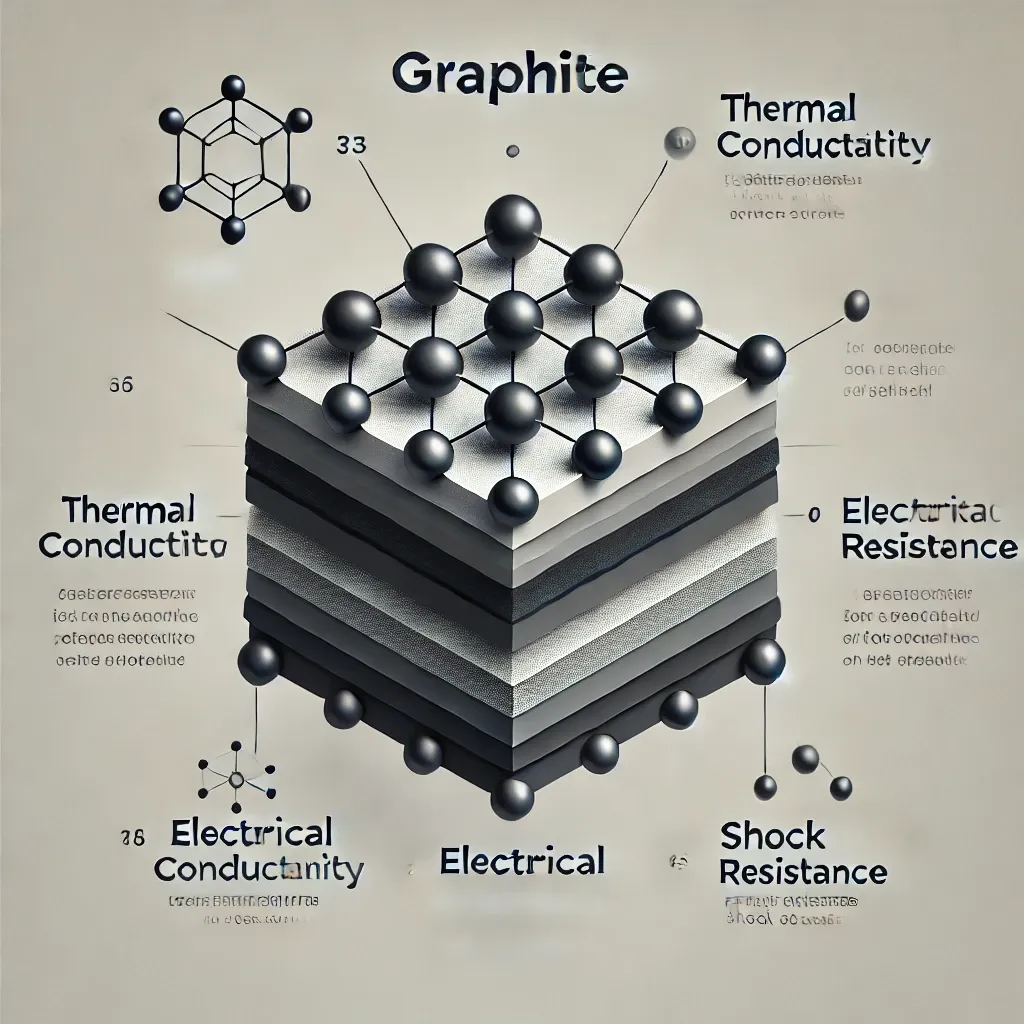
Graphite is a naturally occurring form of crystalline carbon. Structurally, it’s made up of layers of graphene, with each layer consisting of carbon atoms bonded in a hexagonal pattern. What makes graphite unique is the arrangement of these layers: the bonds within each layer are strong, but the bonds between layers are weak, allowing them to slide past each other easily. This characteristic gives graphite its slippery texture, making it a great dry lubricant.
Graphite can be found in nature or synthesized using the Acheson process, which involves heating carbon materials to extremely high temperatures over weeks. Synthetic graphite allows for greater control over specific properties, which is useful for high-tech applications.
2. Material and Mechanical Properties of Graphite
Density and Porosity: Graphite’s density typically ranges from 1.9 to 2.3 g/cm³. Higher density often means better strength and hardness. The porosity of graphite can also be modified to make it impermeable for specific applications.
Hardness and Wear Resistance: On the Mohs scale, graphite has a hardness of 1-2, making it relatively soft. However, it offers good wear resistance, which is why it’s used in applications like bearings and seals.
Strength: Graphite’s strength varies based on the type of force applied:
Compressive Strength: Graphite can handle significant pressure without deforming, especially synthetic graphite.
Flexural Strength: It can withstand bending forces well.
Tensile Strength: Graphite can handle a pulling force due to its layered structure.
Modulus of Elasticity: Graphite is flexible, with a low modulus of elasticity, meaning it can resist deformation without breaking. This flexibility depends on the orientation and treatment of the graphene layers.
3. Thermal Properties of Graphite
One of graphite’s standout properties is its ability to conduct heat, making it useful in high-temperature environments.
Thermal Conductivity: Graphite’s thermal conductivity is governed by the interaction of phonons (vibrations in the material). Pyrolytic graphite, for example, has a much higher thermal conductivity than polycrystalline graphite.
Coefficient of Thermal Expansion (CTE): Graphite has a low CTE, which means it won’t expand or contract significantly with temperature changes. This stability makes it ideal for high-temperature applications.
Thermal Shock Resistance: Graphite can handle rapid temperature changes without cracking, which is crucial in aerospace applications like rocket nozzles.
Heat Capacity: Graphite’s heat capacity allows it to absorb large amounts of heat, making it useful for thermal management applications.
4. Electrical Properties of Graphite
Graphite is also an excellent electrical conductor, thanks to the delocalized electrons in each graphene layer that can move freely throughout the material. This property makes graphite a popular choice for battery anodes, especially in lithium-ion batteries. Its electrical resistivity can vary based on temperature and density, making it adaptable for different electronic uses.
5. Manufacturing and Processing of Graphite
Graphite can be mined naturally or synthesized. The Acheson process allows for precise control over graphite’s properties, such as isotropy. Isotropic graphite has consistent properties in all directions, which is crucial for applications demanding uniform performance, like nuclear reactors. Conventional graphite, on the other hand, is anisotropic, with properties that vary depending on the direction.
6. Applications of Graphite in Industry
Graphite’s unique combination of properties—thermal stability, electrical conductivity, and lubricating ability—makes it essential in various industries:
Refractories: Graphite is used to create heat-resistant materials, such as crucibles and bricks for furnaces.
Batteries: As a key component in lithium-ion batteries, graphite serves as the anode material, contributing to the battery’s long life and efficiency.
Steelmaking: Graphite is used as a carbon additive, improving the strength and quality of steel.
Brake Linings: Graphite’s heat resistance and slipperiness make it ideal for brake linings, where friction is high.
Foundry Facings: Graphite coatings on molds help prevent molten metal from sticking, improving production quality.
Lubricants: Graphite’s slippery layers make it an excellent dry lubricant for machinery.
Nuclear Reactors: Special grades of synthetic graphite, known as nuclear graphite, are used as moderators in nuclear reactors.
7. Advanced Concepts in Graphite Technology
For those interested in a deeper dive, graphite’s properties can be tailored based on particle size, orientation, and processing methods. For example, finer-particle graphite may offer higher strength but less thermal shock resistance. In high-tech industries, isotropy (consistent properties in all directions) is a crucial factor in choosing the right graphite.
Engineers and researchers continuously explore ways to enhance graphite’s performance through advanced processing techniques. Innovations in high-strength graphite materials are paving the way for lighter, more efficient aerospace components and improved thermal management in electronics.
Conclusion
Graphite is a versatile and high-performance material that bridges the gap between everyday products and advanced technology. Its low cost, thermal and chemical stability, and unique structural properties make it a valuable material across numerous industries. As research and innovation continue, we can expect to see graphite play an even greater role in applications from sustainable energy to space exploration. Whether you’re an engineer, scientist, or just curious, graphite’s story is one of resilience and potential—proof that even the simplest materials can shape the future.
This blog post provides an accessible yet detailed overview of graphite, balancing fundamentals with advanced insights to cater to both beginners and those with a technical background.