Atoms: The Building Blocks of the Universe
Podcast
Why Do Atoms Matter?
Understanding atoms is like holding the key to unlock the secrets of the universe. Atoms are fundamental to nearly every scientific field:
- Chemistry: Atoms bond to form molecules, which are the building blocks of compounds. Water, for example, is a molecule made up of two hydrogen atoms and one oxygen atom.
- Physics: The study of atoms helps us understand the nature of matter and energy, and how they interact.
- Biology: Atoms make up the molecules essential for life, like DNA and proteins.
- Materials Science: By manipulating atoms, scientists create new materials with incredible properties—like stronger metals or better conductors.
- Nuclear Energy: Understanding the structure of atoms enables us to harness nuclear energy, which powers entire cities.
What is an Atom?
An atom is the most basic unit of any element, and it's what makes up all the substances we see, touch, and even breathe. You can think of an atom as a tiny Lego brick, and just like how you use Lego bricks to build cars or castles, atoms come together to make up everything in the world—from a grain of sand to a mighty oak tree, and even the stars in the night sky.
Atoms are incredibly small—so small that millions of them would fit on the head of a pin. Yet, despite their tiny size, atoms are incredibly complex and fascinating.
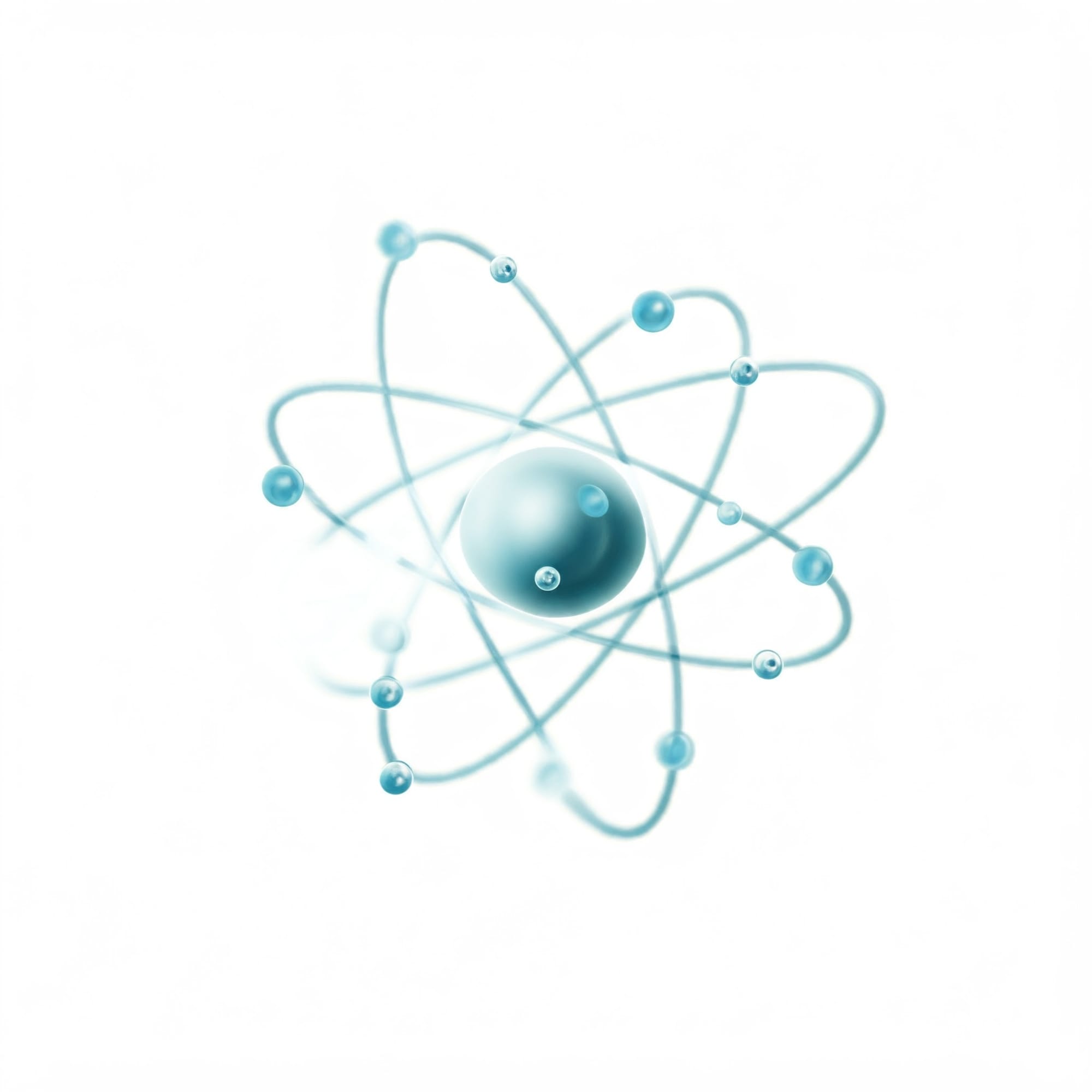
The Structure of an Atom
At the core of every atom is the nucleus, a dense central region that holds most of the atom's mass. This nucleus contains two types of subatomic particles:
- Protons: Positively charged particles that determine the identity of the atom. For instance, every hydrogen atom has exactly one proton.
- Neutrons: Neutral particles that add mass but no charge to the nucleus.
Surrounding this nucleus, we have electrons. These tiny, negatively charged particles are in constant motion, orbiting the nucleus at astonishing speeds. Imagine bees buzzing around a flower—electrons move even faster! These electrons are crucial because their arrangement determines an atom's chemical properties and how it bonds with other atoms.
Elements and the Periodic Table
Atoms are the building blocks of elements. Each element on the periodic table is defined by the number of protons in its nucleus. This number is called the atomic number. For example, hydrogen, the lightest and most abundant element in the universe, has one proton. Helium has two, carbon has six, and so on. The periodic table is like a catalog of all the building blocks available for creating the universe. Each element has unique properties, and by combining them in different ways, we get the incredible variety of substances around us.
Isotopes: The Unique Versions of Elements
Not all atoms of an element are identical—some have extra neutrons. These variations are called isotopes. Isotopes of an element have the same number of protons but different numbers of neutrons. For example, carbon-12 and carbon-14 are both carbon, but they have different masses due to the differing number of neutrons. Some isotopes are stable, while others are radioactive and decay over time, releasing energy. This property has many applications, from medical imaging to determining the age of ancient artifacts.
The Quantum World: Stranger Than Fiction
If you think atoms are fascinating, wait until you hear about the quantum world. The behavior of atoms is governed by the rules of quantum mechanics—a set of principles that are often counterintuitive and strange. Electrons, for example, can exist in multiple places at once, and they don't move in the way we typically understand motion. Quantum mechanics has revolutionized our understanding of matter and energy, leading to incredible technological advancements like lasers, MRI machines, and transistors (which are the basis of all modern electronics).
In Conclusion
Atoms are the tiny building blocks that make up everything we see around us, and they are much more than just simple particles. They are the key to understanding the structure of the universe, the nature of matter, and the mysteries of energy. By studying atoms, we can unlock new technologies, gain deeper insights into the world, and truly appreciate the beauty of everything that makes up our reality. Whether it's the air we breathe or the stars in the sky, it all starts with atoms—the humble yet magnificent building blocks of the universe.